Abstract
Developing high-performance porous materials to separate ethane from ethylene is an important but challenging task in the chemical industry, given their similar sizes and physicochemical properties. Herein, a new type of ultra-strong C2H6 nano-trap, CuIn(3-ain)4 is presented, which utilizes multiple guest-host interactions to efficiently capture C2H6 molecules and separate mixtures of C2H6 and C2H4. The ultra-strong C2H6 nano-trap exhibits the high C2H6 (2.38 mmol g−1) uptake at 6.25 kPa and 298 K and demonstrates a remarkable selectivity of 3.42 for C2H6/C2H4 (10:90). Additionally, equimolar C2H6/C2H4 exhibited a superior high separation potential ∆Q (2286 mmol L−1) at 298 K. Kinetic adsorption tests demonstrated that CuIn(3-ain)4 has a high adsorption rate for C2H6, establishing it as a new benchmark material for the capture of C2H6 and the separation of C2H6/C2H4. Notably, this exceptional performance is maintained even at a higher temperature of 333 K, a phenomenon not observed before. Theoretical simulations and single-crystal X-ray diffraction provide critical insights into how selective adsorption properties can be tuned by manipulating pore dimensions and geometry. The excellent separation performance of CuIn(3-ain)4 has been confirmed through breakthrough experiments for C2H6/C2H4 gas mixtures.
Background
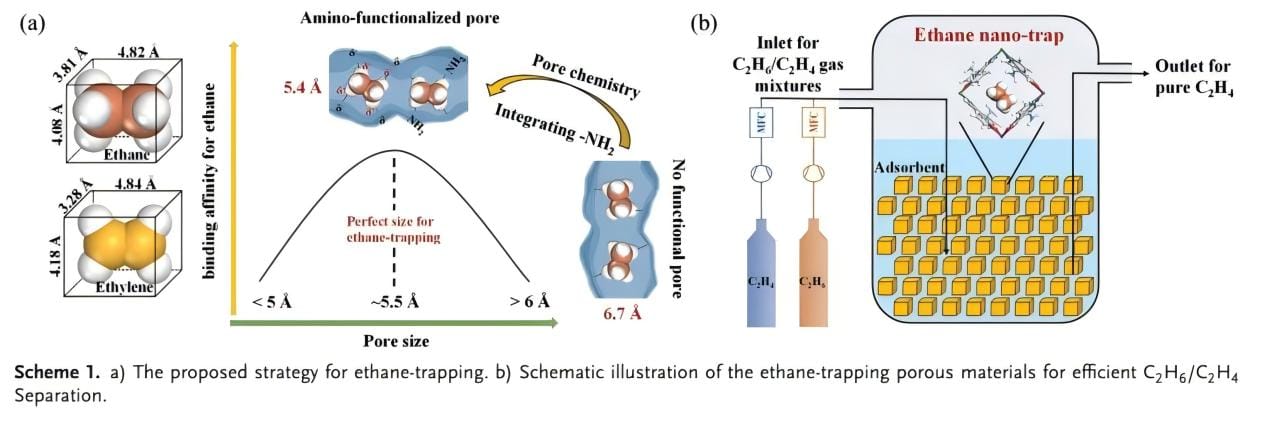
Traditional industrial methods for separating ethane and ethylene have defects such as high energy consumption and high investment, so it is urgent to develop a green, energy-saving and economical method to replace it. The adsorption separation technology of porous materials has the potential advantages of low energy consumption and low investment. Among them, MOFs, due to its unique pore structure, has become one of the important materials in the adsorption separation process. In the field of ethylene and ethane separation, ethane-selective MOFs can reduce the desorption energy consumption and achieve one-step purification of ethylene. However, the MOFs materials reported so far have problems such as limited adsorption capacity and insufficient selectivity. On the other hand, in actual industrial scenarios, the temperature of the mixed gas is usually higher than the ambient temperature, and the separation of olefins and alkanes at high temperatures is conducive to improving efficiency and saving energy. Therefore, it is crucial to evaluate the performance of the adsorbent under conditions that are closer to the simulated industrial environment. However, the performance of C2H6-selective MOFs at higher temperatures has rarely been studied and realized. Therefore, it is imperative to develop an adsorbent that can effectively separate C2H6/C2H4 mixtures at high temperatures. In this paper, amino functionalization is used to precisely control the pore environment to achieve excellent C2H6/C2H4 separation (Scheme 1).
Material synthesis and structure
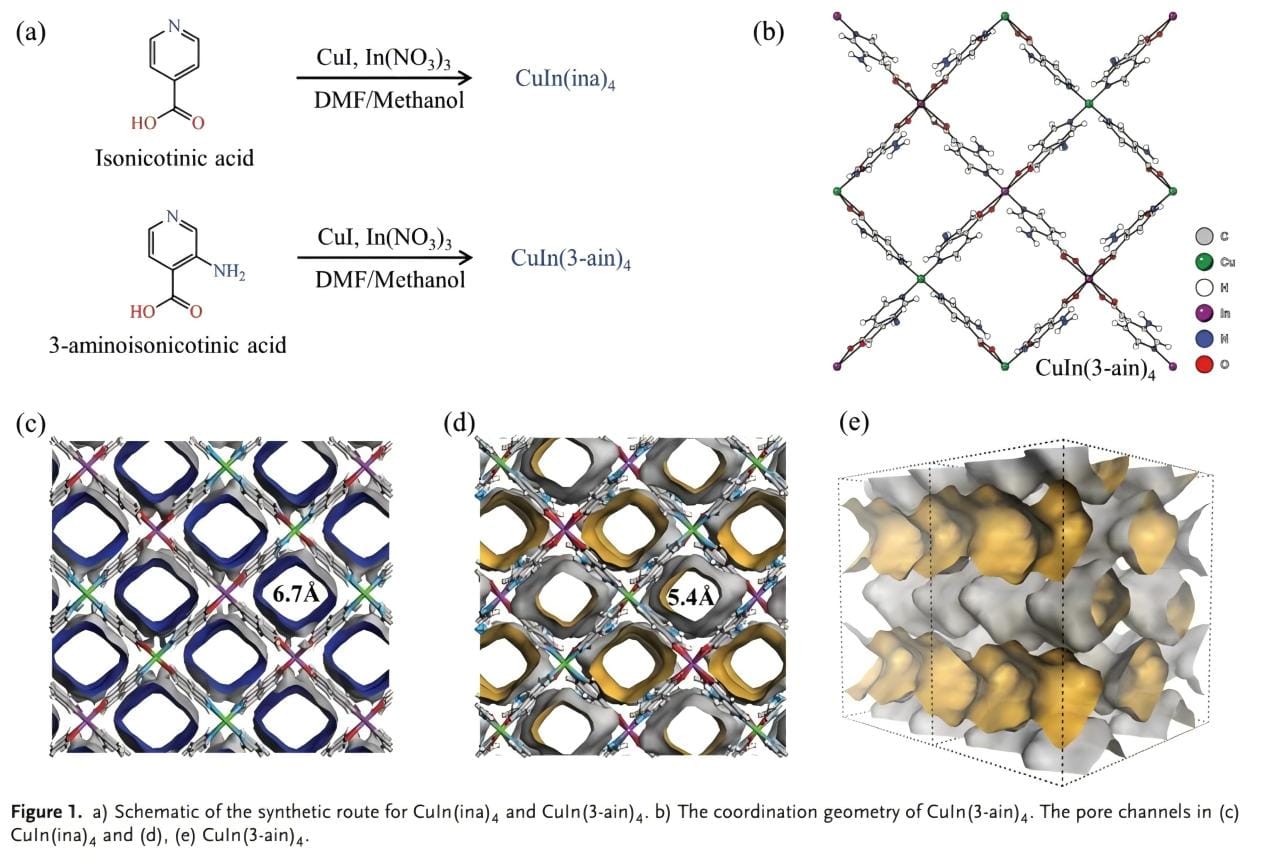
CuIn(ina)4 is a doubly interpenetrating diamond (dia) network with square channels extending along the a-axis. In a mixture of N,N-diethylformamide (DEF)/methanol, CuI, In(NO3)3·xH2O and 3-aminoisonicotinic acid were subjected to a hot solvothermal reaction at 100°C to generate orange crystals of CuIn(3-ain)4. Single crystal X-ray diffraction analysis showed that the CuIn(3-ain)4 crystals belong to the orthorhombic Fdd2 space group. The structure consists of two different tetrahedral coordination units [CuN4]+ and [In(COO)4]−, connected by 3-aminoisonicotinic acid. The Cu+ ions are tetrahedrally coordinated by the pyridine nitrogen atoms of four independent 3-aminoisonicotinic acid ligands, while the In3+ ions are chelated by the carboxylic acid groups of four ligands, following the soft and hard acid-base theory, to construct an open three-dimensional neutral framework with a rhombohedral topology. Compared with CuIn(ina)4, the introduction of amino groups can effectively reduce the size of accessible pore space in certain directions and help to form a pore surface with more interaction sites.
Adsorption behavior
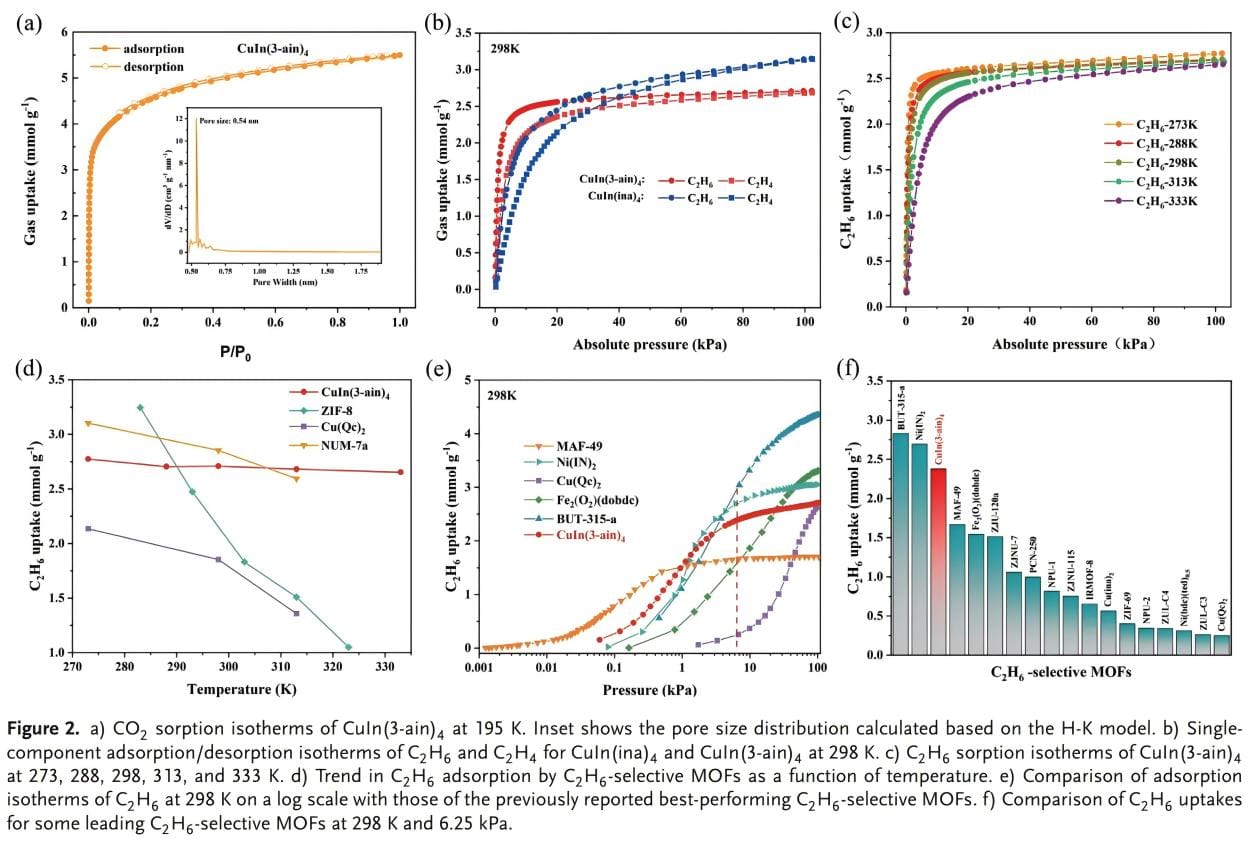
The permanent porosity of CuIn(ina)4 and CuIn(3-ain)4 was verified by the CO2 adsorption isotherm at 195 K (Figure 2a). Both materials showed typical type I adsorption isotherms. The (BET) surface areas of CuIn(ina)4 and CuIn(3-ain)4 were determined to be 472 m2/g and 429 m2/g, respectively. Based on the H-K method, the pore size distribution (PSD) of CuIn(ina)4 was calculated to be 0.67 nm. After integrating -NH2, the PSD of CuIn(3-ain)4 was reduced to 0.54 nm. The adsorption isotherms at 298K show (Figure 2b) that the adsorption isotherms of CuIn(3-ain)4 for C2H6 and C2H4 after amino functionalization have significantly increased steepness, with the adsorption amount of C2H4 being 2.68 mmol/g and the adsorption amount of C2H6 being 2.71 mmol/g (Figure 2b). At 313K and 333K, the adsorption values of CuIn(3-ain)4 for C2H6 were 2.68 and 2.65 mmol/g, respectively, and there was no obvious decrease in the adsorption amount. This special performance is rarely observed in other C2H6 selective MOF adsorbents (Figure 2d). And at a low pressure of 6.25 kPa, the adsorption amount of CuIn(3-ain)4 can reach 2.38 mmol/g, which exceeds most existing adsorbents. The results show that CuIn(3-ain)4 has great potential in separating C2H6/C2H4.
IAST and separation potential
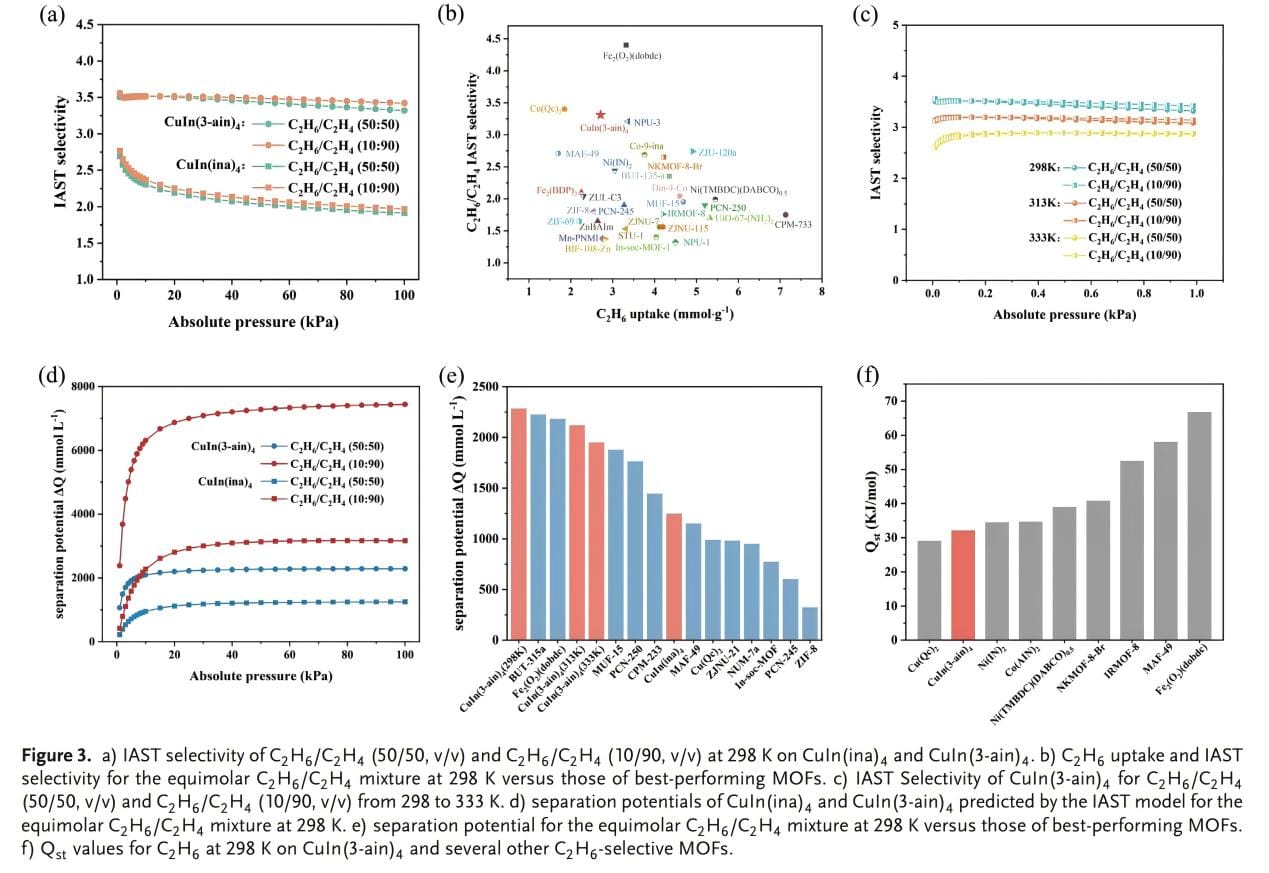
The adsorption selectivity of C2H6/C2H4 binary mixtures at 298 K was further quantified using ideal adsorption solution theory (IAST). In C2H6/C2H4 mixtures (50/50 and 10/90), the IAST selectivities of CuIn(ina)4 were 1.91 and 1.97, respectively. CuIn(3-ain)4 showed higher selectivities for the same mixtures, 3.32 and 3.42, respectively (Figure 3a). At 1 bar, the adsorption of C2H6 exceeded 2.68 mmol/g, while the separation selectivity of C2H6/C2H4 (50/50) exceeding 3.32 was extremely rare (Figure 3b). In addition, it remained at 3.08 and 2.86 at 313 and 333 K, respectively (Figure 3c), which is suitable for industrial applications. The separation potential ∆Q was further calculated to estimate the C2H6/C2H4 separation ability of CuIn(3-ain)4 in a fixed bed adsorber (Figure 3d). The results showed that the maximum recovery of CuIn(3-ain)4 for equimolar C2H6/C2H4 can reach 2286 mmol/L at room temperature; this value significantly exceeds that of CuIn(ina)4, which may represent the highest separation potential to date. Qst calculations showed that CuIn(3-ain)4 exhibited a significantly lower Qst value (32.11 kJ/mol) compared to other materials, indicating a high regeneration capacity under mild conditions.
Dynamic Adsorption
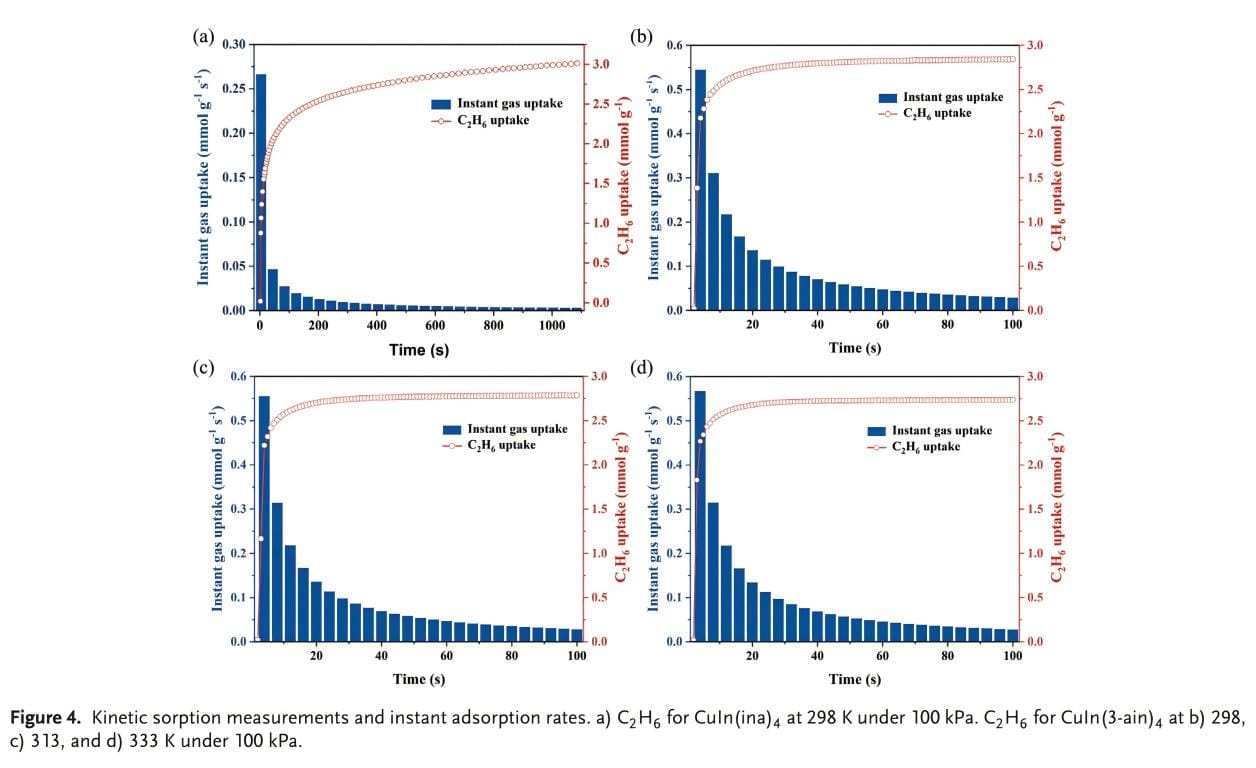
The mass transfer effect between gas adsorbate and adsorbent at 298 K and 100 kPa was investigated by gas dynamic adsorption experiments. As shown in Figure 4a, b, the maximum adsorption rates of CuIn(ina)4 and CuIn(3-ain)4 for C2H6 are 0.27 and 0.54 mmol/g/s, respectively. It is worth noting that the maximum adsorption rate of CuIn(3-ain)4 is twice that of CuIn(ina)4. This difference can be attributed to the optimal pore size and the presence of internal amine functional groups to enhance the diffusion and capture of ethane. In addition, the adsorption rate of CuIn(3-ain)4 for C2H6 is higher than that for C2H4, further verifying the high affinity of the material for C2H6. In addition, the adsorption rate curves of CuIn(3-ain)4 for ethane at 313 and 333 K and 100 kPa were studied to evaluate the effect of temperature on adsorption kinetics (Figure 4c, d). The experiments showed that the adsorption rate of CuIn(3-ain)4 for C2H6 increased with increasing temperature. This helps to maintain the high adsorption capacity of CuIn(3-ain)4 at high temperatures. These results show that the adsorption of gases in these two MOFs is not only affected by thermodynamic factors, but also by kinetic adsorption effects, which has a synergistic effect on the entire adsorption process.
Adsorption site
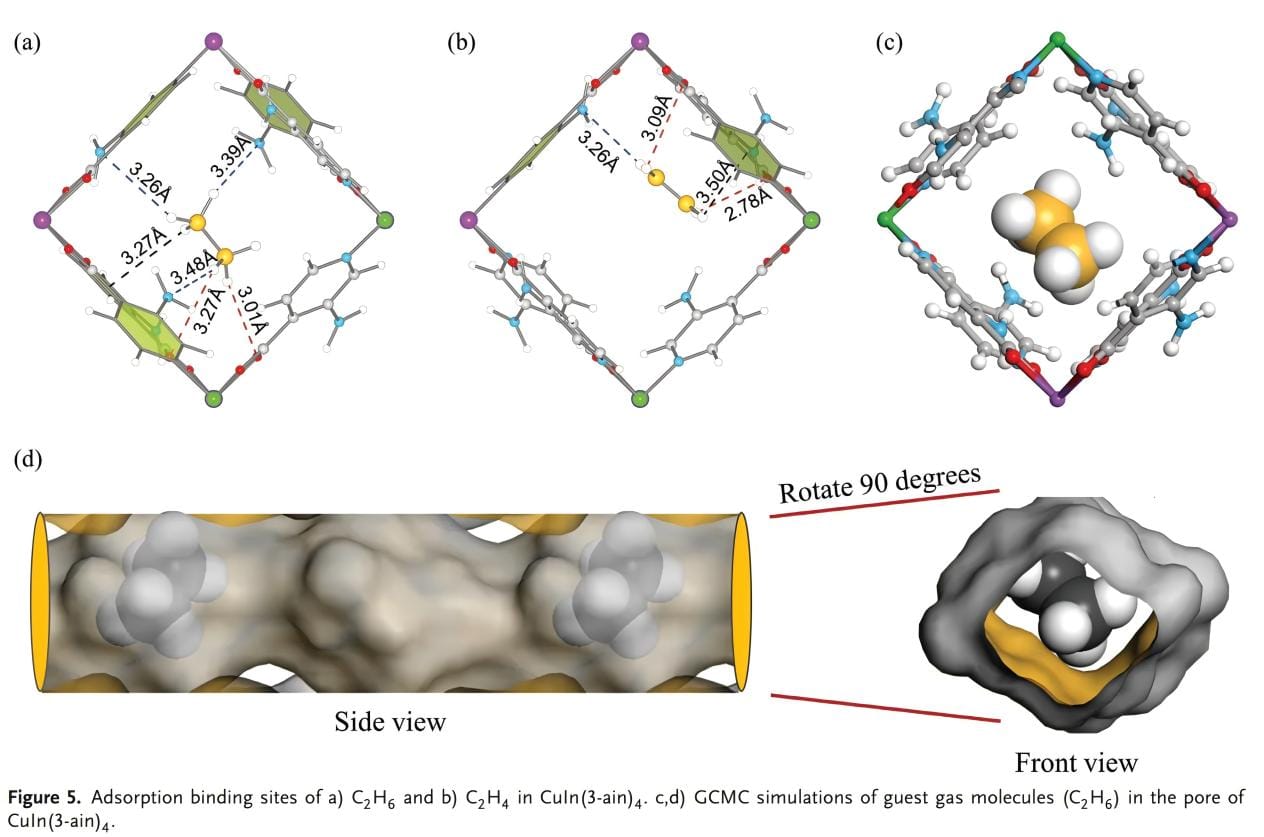
The binding interactions between gas molecules and the host framework were investigated by Grand Canonical Monte Carlo (GCMC) simulations and DFT calculations. As shown in Figure 5a, b, C2H6 molecules produce a variety of supramolecular interactions with the framework. Each C2H6 molecule is connected to the surrounding carboxylic acid oxygen atoms by H bonds through two C-H··O interactions at a distance of 3.01 to 3.27 Å, and forms a C─H···Π interaction with the pyridine ring at 3.27 Å. In addition, the C2H6 molecule interacts with the nitrogen-containing groups to form three strong C─H···N hydrogen bonds, while the adsorbed C2H4 molecules interact less with the framework at a longer distance, mainly interacting with the framework through C─H··O interactions (2.78 to 3.09 Å), C─H···N interactions (3.50 Å) and C─H···N interactions (3.26 Å). The change in bond length confirms that the addition of amino groups changes the host-guest interaction dynamics, and the interaction between amino groups and hydrogen atoms enhances the adsorption of C2H6. The temperature insensitivity of CuIn(3-ain)4 to C2H6 mainly depends on the synergistic effect of multiple supramolecular interactions. Figure 5c, d shows the simulated spatial distribution of guest gas molecules (C2H6) in CuIn(3-ain)4.
Dynamic penetration test
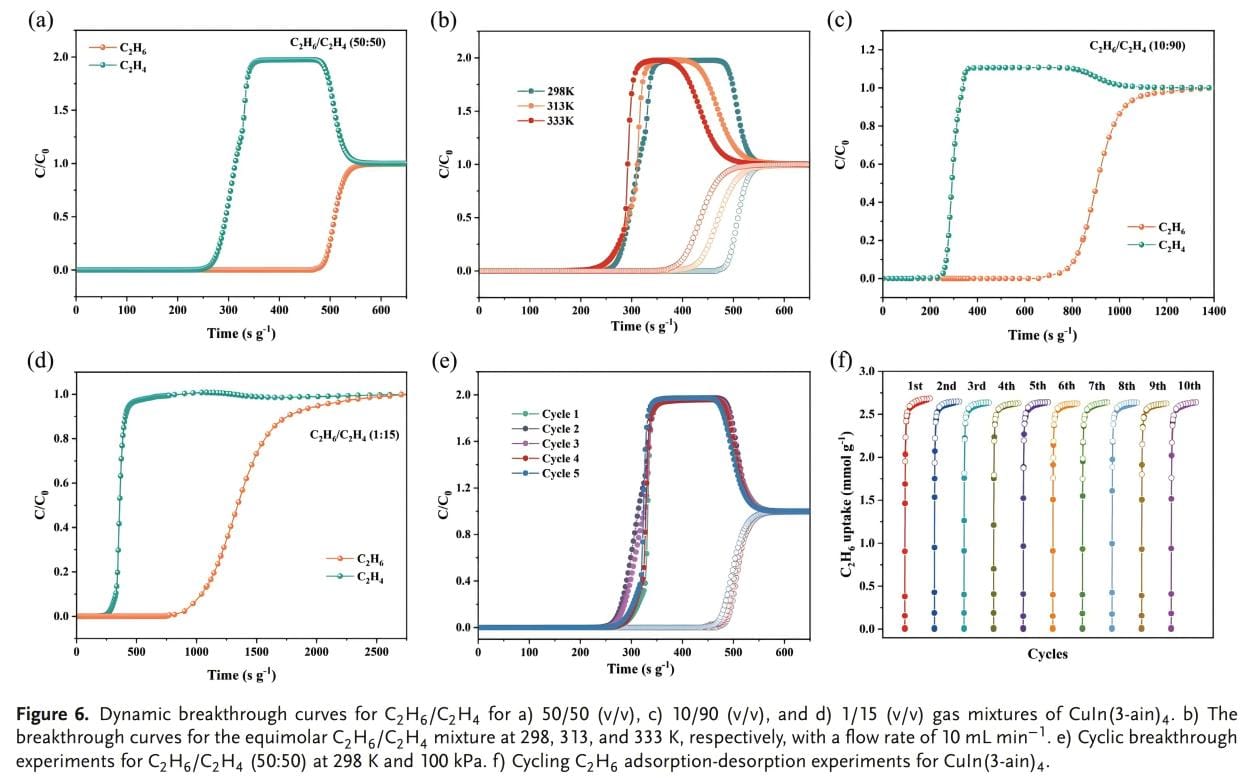
In order to evaluate the separation effect of CuIn(3-ain)4 on the C2H6/C2H4 mixture, penetration experiments were conducted in the temperature range of 298 ~ 333 K. Figure 6a shows that CuIn(3-ain)4 can effectively separate the C2H6/C2H4 mixture at 298K. During the initial purge process, C2H4 (>99.95%) is first detected, and the retention time of C2H6 is 267 s/g. A single penetration can recover 7.64 L/kg C2H4 (purity ≥99.5%) from the C2H6/C2H4 (50/50) mixture. Figure 6b further confirms the adaptability of CuIn(3-ain)4 in a wide temperature range. This article conducted penetration experiments on actual industrial scenarios, namely C2H6/C2H4 (10/90 and 1/15) mixtures. This further proves that CuIn(3-ain)4 can effectively purify C2H4 even at low concentrations of C2H6. From the breakthrough curve of C2H6/C2H4 (10/90), the dynamic adsorption amounts of C2H6 and C2H4 were determined to be 0.68 and 1.55 mmol/g respectively. The dynamic separation selectivity of C2H6/C2H4 is 3.95, which is significantly higher than the static selectivity of 3.42, indicating that CuIn(3-ain)4 has excellent dynamic separation performance. CuIn(3-ain)4 can still maintain its original dynamic separation performance after multiple cycles of dynamic penetration experiments. And the estimated cost of CuIn(3-ain)4 is ≈$40/kg. CuIn(3-ain)4 has excellent separation efficiency, durability and cost-effectiveness, and is expected to be applied in practical industries.
Summary and prospec
In summary, a new ethane selective adsorption material CuIn(3-ain)4 was successfully synthesized by introducing amino groups. The material has a strong adsorption affinity for ethane, reaches saturation at low pressure, and has excellent separation performance for C2H6/C2H4 mixtures, with high separation potential among MOF materials. The C2H6/C2H4-IAST selectivity of CuIn(3-ain)4 is superior to most known C2H6 selective MOF materials. In addition, the adsorption capacity and separation potential of CuIn(3-ain)4 remain consistent over a wide temperature range, making it very suitable for ethylene purification. Since the separation process only partially depends on thermodynamics, the synergistic effects of thermodynamics and kinetics can be exploited to effectively and selectively adsorb C2H6 at moderate Qst. Dynamic breakthrough experiments confirm the excellent separation performance of CuIn(3ain)4 for C2H6/C2H4. Theoretical simulations provide insights into how to fine-tune the selective adsorption properties of the adsorbent by controlling the pore size and geometry. The successful application of CuIn(3-ain)4 in C2H6/C2H4 separation provides a new reference for the design of C2H6-selective MOFs and inspires further investigation of its potential in other complex alkane/alkene separation tasks.
Article link: https://doi.org/10.1002/smll.202402382